Florian Group
Stem Cell Aging
Understanding alterations of aged somatic stem cells and improve the regeneration of tissues
Aging is the first risk factor for most diseases and we believe that understanding the biology of stem cell aging will be critical to enhance stem cell function and preserve their regenerative capacity over time. Our mission is to end the threat of age-related diseases and promote longevity

Lab focus – Rejuvenating stem cells
Somatic stem cells have the ability to regenerate tissues over time. This
capacity declines with age, disrupting tissue maintenance. Many age-related
diseases, including cancer, immunosenescence and sarcopenia, are likely
consequence of stem cell dysfunction. Understanding how stem cells age and
identifying intervention strategies to maintain stem cell function and
regenerative capacity will lead to new therapeutic approaches for maintaining
our health as we age. Our lab uses mouse models to explore the biology of
somatic stem cell function and regeneration and to identify mechanisms of
age-related stem cell dysfunction. Our focus is on epigenetic alterations that
can be pharmacologically targeted and on the interactions between stem cells
and the niche. Based on our findings we have identified intervention strategies
to improve the regenerative capacity of aged stem cells and we have
unravelled biological alterations in the stem cell niche that could help our
understanding of disease development in the elderly.
Why it matters
Interventions that rejuvenate stem cells are likely to provide new avenues to
prolong human health- and lifespan and treat or prevent a wide range of age-
related diseases. Coupling fundamental insight into stem cell biology to the
exploration of such intervention strategies will allow for the identification of new
promising therapeutic approaches.
News
 M Carolina Florian new ICREA Research Professor
ICREA, the Catalan Institution for Research and Advanced Studies, offers permanent, tenured positions to the most talented and extraordinary scientists and academics to come and work in Catalonia.
Read Article
M Carolina Florian new ICREA Research Professor
ICREA, the Catalan Institution for Research and Advanced Studies, offers permanent, tenured positions to the most talented and extraordinary scientists and academics to come and work in Catalonia.
Read Article  The team led by Dr. M. Carolina Florian at IDIBELL receives a European grant of 2 million euros to study bone marrow rejuvenation
The team led by Dr. M. Carolina Florian at IDIBELL receives a European grant of 2 million euros to study bone marrow rejuvenation
With this project, Dr. M Carolina Florian, from the IDIBELL’s Regenerative Medicine Program, aims to improve the survival and quality of life of elderly patients undergoing chemotherapy
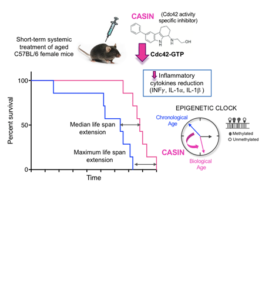 Inhibition of Cdc42 activity extends murine lifespan
Inhibition of Cdc42 activity extends murine lifespan
Cdc42 is a small RhoGTPase regulating multiple functions in eukaryotic cells…
 La inteligencia artificial, nueva aliada contra la leucemia
La inteligencia artificial, nueva aliada contra la leucemia
La leucemia mieloide aguda es un tipo de cáncer de la sangre muy agresivo. Afecta 10 veces……
Job openings
Predoctoral Researcher to Join Dr. Florian’s Lab
Application submission deadline:
28/02/2022
The candidate will be involved in an international research team focusing on investigating regeneration of endothelial cells and vessels in the bone marrow. The team aims at developing and implementing cutting-edge technologies (3D-whole mount bone marrow microscopy analysis, single cell transplantation, single cell RNA-seq and ATAC-seq, deep learning-based multivariate data analyses) to identify how vessels regenerate in the bone marrow.
Funding
Keywords
Stem Cells, Aging, Regenerative Medicine, Epigenetics, Chromatin, Hematopoietic Stem Cell, Bone Marrow Niche
Publications
Transplanting rejuvenated blood stem cells extends lifespan of aged immunocompromised mice.
Montserrat-Vazquez S, Ali NJ, Matteini F, Lozano J, Zhaowei T, Mejia-Ramirez E, Marka G, Vollmer A, Soller K, Sacma M, Sakk V, Mularoni L, Mallm JP, Plass M, Zheng Y, Geiger H, Florian MC.NPJ Regen Med. 2022 Dec 29;7(1):78. doi: 10.1038/s41536-022-00275-y.
Aging of the Hematopoietic Stem Cell Niche: New Tools to Answer an Old Question.
Matteini F, Mulaw MA, Florian MC.Front Immunol. 2021 Nov 11;12:738204. doi: 10.3389/fimmu.2021.738204. eCollection 2021.
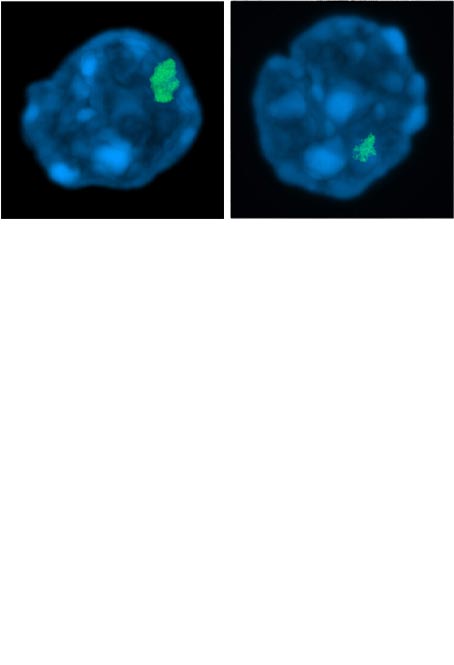
Grigoryan A, Pospiech J, Krämer S, Lipka D, Liehr T, Geiger H, Kimura H, Mulaw MA, Florian MC. (2021) Attrition of X Chromosome Inactivation in Aged Hematopoietic Stem Cells. Stem Cell Reports 16(4):708-716.
Florian MC, Leins H, Gobs M, Han Y, Marka G, Soller K, Vollmer A, Sakk V,
Nattamai KJ, Rayes A, Zhao X, Setchell K, Mulaw M, Wagner W, Zheng Y, Geiger
H. (2020) Inhibition of Cdc42 activity extends lifespan and decreases circulatinginflammatory cytokines in aged female C57BL/6 mice. Aging Cell 19(9):e13208.
Mejia-Ramirez E, Florian MC. (2020) Understanding intrinsic hematopoietic stemcell aging. Haematologica 105(1):22-37.
Zjablovskaja P, Florian MC. (2019) Acute Myeloid Leukemia: Aging and Epigenetics. Cancers (Basel) 12(1):103.
Sacma M, Pospiech J, Bogeska R, de Back W, Mallm JP, Sakk V, Soller K, Marka G, Vollmer A, Karns R, Cabezas-Wallscheid N, Trumpp A, Mendez-Ferrer S, Milsom MD, Mulaw M, Geiger H, Florian MC (2019) Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat Cell Biol in press
Grigoryan A, Guidi N, Senger K, Liehr T, Soller K, Marka G, Vollmer A, Markaki Y, Leonhardt H, Buske C, Lipka DB, Plass C, Zheng Y, Mulaw MA, Geiger H, Florian MC. (2018) LaminA/C regulates epigenetic and chromatin architecture changes upon aging of hematopoietic stem cells. Genome Biol 19(1):189.
Florian MC*, Sacma M, Klose M, Knudson L, Soller K, Marka G, Nattamai KJ, Sakk V, Zheng Y, Mulaw MA, Glauche I, Geiger H* (2018) Aging alters the epigenetic asymmetry of HSC division. DOI 10.1371/journal.pbio.2003389. PLOS Biology *corresponding authors
Florian MC, Klenk J, Marka G, Soller K, Kiryakos H, Peter R, Herbolsheimer F, Rothenbacher D, Denkinger M, Geiger H (2017) Expression and activity of the small RhoGTPase Cdc42 in blood cells of older adults are associated with age and cardiovascular disease. J Gerontol A Biol Sci Med Sci
Florian MC, Nattamai KJ, Dorr K, Marka G, Uberle B, Vas V, Eckl C, Andra I, Schiemann M, Oostendorp RA, Scharffetter-Kochanek K, Kestler HA, Zheng Y, Geiger H (2013) A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 503: 392-6
Members
Past Members
Ani Grigoryan
Johannes Pospiech
Noelle J. Ali
Polina Zjablovskaja
Francesco Maria Affuso
Barbara Walter
Johannes Pospiech
Noelle J. Ali
Polina Zjablovskaja
Francesco Maria Affuso
Barbara Walter