P-CMR[C] researchers define a new mechanism of aging in a bone marrow stem cell in women
April 7, 2021
- To equilibrate the gene expression between XX females and XY males, one of female X chromosomes is inactivated.
- Researchers from IDIBELL Regenerative Program has discovered that this inactivation is affected in bone marrow aged stem cells.
- The study, published in Stem Cell Reports, also suggest that impairment in female chromosome X inactivation could be related with a group of blood cells cancers.
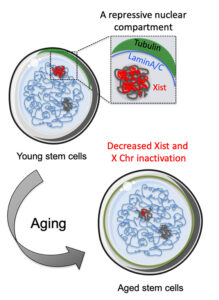
In women cells, the expression of chromosome X is silenced to balance gene expression between XX females and XY males. One of the most general mechanisms for gene expression regulation is the distribution of DNA inside the nucleus. In mammalian cells, we distinguish two compartments: euchromatin, located at the center of the nucleus, where the DNA is more relaxed and, the genes presented a high rate of expression; and heterochromatin, at the periphery of the nucleus with compacted DNA and silenced gene expression.
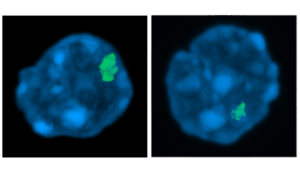
A study led by Dr. Maria Carolina Florian, from P-CMR[C] and IDIBELL Stem Cell Aging research group, and Dr. Medhanie A. Mullaw, from University Hospital Ulm, has demonstrated that the nuclear protein Lamin A/C creates a compartment in the nucleus of young bone marrow stem cells to inactive the chromosome X in female cells.
The study, published at Stem Cell Report, indicates that in aged bone marrow stem cells, the Lamin A/C inactivating compartment is largely reduced, which impairs the chromosome X periphery localization and inactivation. The observations indicate a general relocalization of the “inactivated” chromosome X to more active regions of the nucleus, which correlates with the higher gene expression of these chromosomes in aged cells.
The deregulation of chromosome X inactivation is strongly associated to myelodysplastic syndrome, a group of cancers more frequently affecting elderly people, in which the blood cells do not mature. Therefore, the existence of an age‐dependent alteration of chromosome X inactivation may raise important implications for understanding the physiology of hematopoiesis during aging and the pathogenesis of age‐related hematopoietic malignancies.