Researchers outline the mechanisms that control the conversion between fetal and adult intestinal stem cells
July 18, 2023
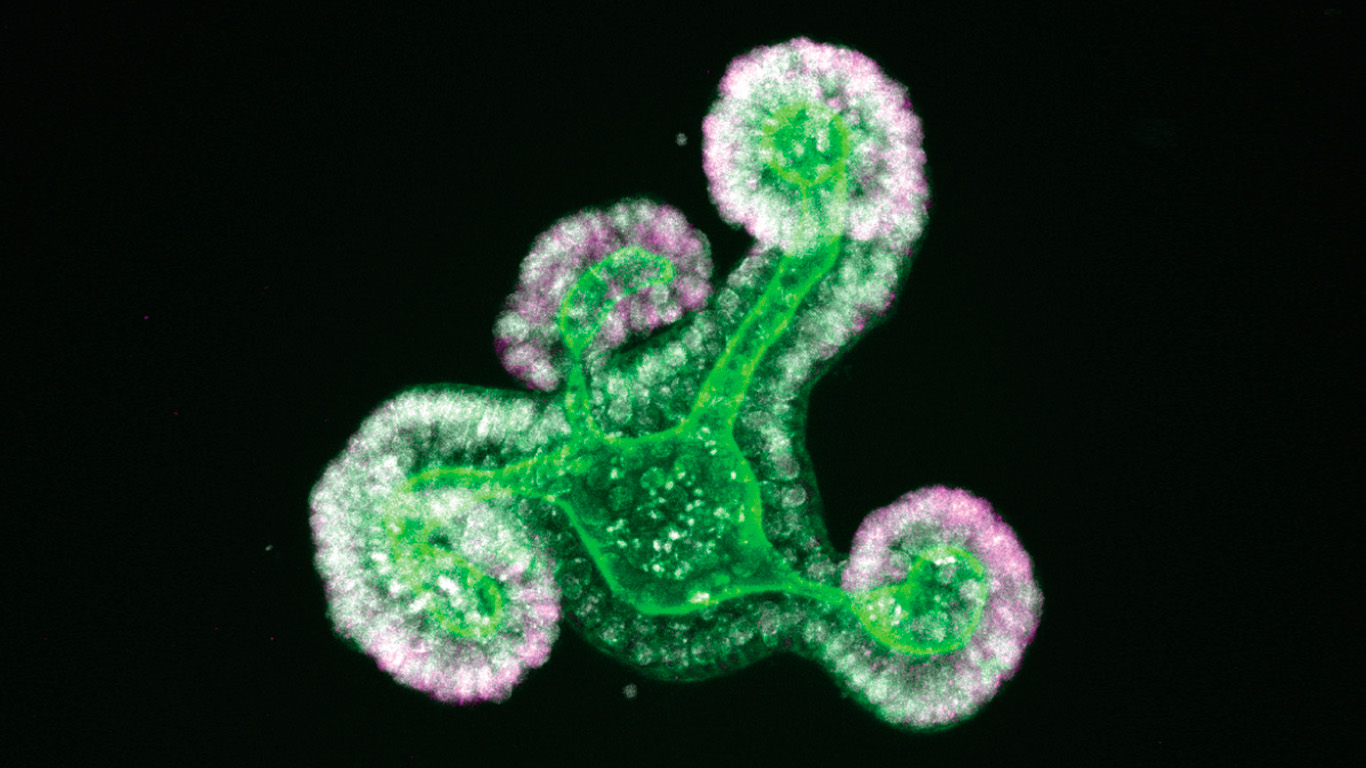
The process by which organs are formed, going from an immature to a fully mature and functional state, is extremely complex and unknown. There are still many cellular and molecular mechanisms that control the process that are unknown. In the case of the intestine, this implies acquiring the capacity to digest and absorb nutrients, a process controlled molecularly by specific transcription factors. These transcription factors are responsible for turning on and off genes that code for proteins that allow cells to transition between fetal or adult stem cells and differentiated cells.
Deciphering the functioning of some transcription factors in the formation of the intestine has been the objective of the team led by Professor Kim Bak Jensen, from the Novo Nordisk Foundation of Stem Cell Medicine (reNEW), in which Dr. Jordi Guiu has also participated, Head of the Cellular Plasticity and Regeneration research group at the Bellvitge Biomedical Research Institute (IDIBELL) and the Clinical Translation Program for Regenerative Medicine of Catalonia (P-CMR[C]). Now, their results are presented in two articles published in the scientific journal Science Advances.
In the two published studies, the scientific team uses the intestine organoid as a model, a three-dimensional structure similar to mini-intestines created in the laboratory from stem cells. Using different molecular approaches, they demonstrate the utility of organoid models to identify the factors that regulate cell fate and reveal that the transcription factors SMARCA4 and SMARCC1 prevent early differentiation of stem cells during intestinal development. In addition, they identify the transcriptional activity of the YAP protein as the main regulator of the immature fetal state.
Dr. Jordi Guiu explains: “Understanding the state transition from an immature to a mature organ is likely to enable us with insight into how we can coach cells or organ-like structures grown in the lab from immature to mature structures. Such knowledge will ultimately help scientists generate cellular therapies, for instance, via the production of epithelial cells in the lab that can be transplanted into patients with ulcerating conditions or other epithelial defects.”

